pH is a vital metric for any hydroponic system, if not the most essential metric. Most importantly it enables your crops to access the nutrients they need.
What Is pH?
pH stands for potential of hydrogen. It is a scale used to specify the acidity or basicity of an aqueous solution. Acidic solutions (solutions with higher contentration of H+ ions) are measuredd to have lower pH values than basic or alkaline solutions. source
More specifically: Water reacts with itself and creates two kinds of molecules: hydroxyl H3O(+) and hydroxide: OH(-). The pH determines the amount of hydroxide ions in the water. So the more hydroxide there is in the water the higher the pH. Also both moledules determine each other’s concentration, because water maintains an equilibria of the both which always sums up to 14. That’s why the pH scale is from 0-14 and when both molecules are in balance the scale indicates a pH of 7, which is neutral. For a more in depth explanation visit Science in Hydroponics.
Why Is pH Important for Hydroponics?
Now, we learned what pH means but there is no connection to hydroponics yet. The pH level has an effect on a wide variety of things but one important effect of pH is the nutrient availability. Meaning the availability of nutrients in hydroponic solutions is depend upon the pH. In the following chart you see which nutrients are available for what pH levels.
The Effect of pH on Nutrient Availability in Hydroponic Solutions with the optimal pH Range for Hydroponics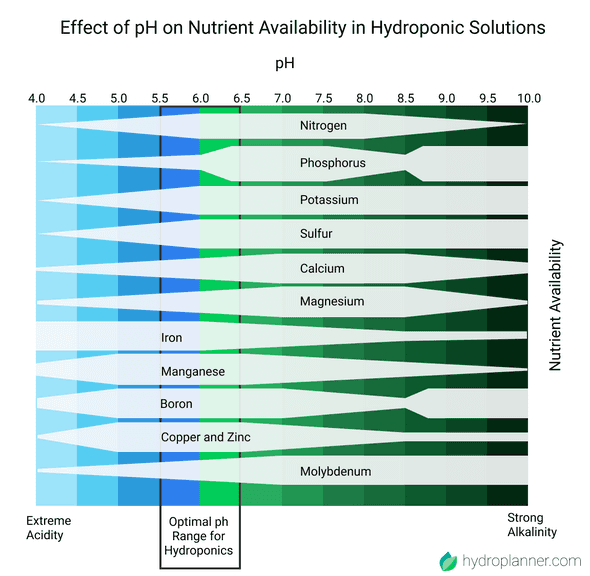
Availability means, that plants can access those nutrients within the solution with their roots. So, if the pH is off, plants cannot access necessary nutrients and hence might not be able to grow properly. Therefore it’s important to maintain the right range of pH depending on the nutrients the plants need. The chart also highlights the optimal range for hydroponic applications which is between 5.5 and 6.5 with slight variations per plant.
Our Solutionexplorer consists of a longer list of plants and their favourite pH levels, it also enables you to seek out the optimal pH range for all the plants you have in your system by simply selecting them.
Measuring pH
We learned what pH actually is and why it is important. Now the next step is to measure the pH to understand what the status quo is. After that, we also look into adjusting the pH in order for us to make it right for the crops. Testing the pH level so measuring the amount of hydroxide ions can be achieve through various different means.
Using A Litmus Test to Measure pH
The easiest and cheapest way to do this, is by using litmus test strips. A litmus test is a small piece of paper which can be emerged into a solution, after that the color of the sensitive dye on the paper will change. Afterwards the color is compared to a scale which is often deliver with the test to determine the pH. It’s very simple and has some advantages but also disadvantages.
Advantages:
- Easy to do
- No calibration needed
- Cheap
Disadvantages:
- Not reusable
- The accuracy of this test is usually bound to integer values
- The reading is dependend on color comparison which can change in different lights
Using a Liquid Testing Kit to Measure pH
The next tier of testing which is a little bit more expensive but also more accurate is a liquid testing kit. This kind of test is usually applied by swimming pool owners. The measurement process works as follows.
- A sample of the solution to be tested is taken
- A few drops of the testing liquid are added
- After a short while, the color of the mix changes
- Comparing the color of the mix to the scale delivered with the testing kit releaves the pH level (similar to litmus test)
Some liquid testing kits are especially made for hydroponics and therefore offer a more fine-grained scale which can be used to determine more accurate pH levels and not only integer numbers.
Advantages:
- Easy to do
- No calibration needed
- Cheap
- More accurate than litmus tests
Disadvantages:
- Not reusable
- The reading is dependend on color comparison which can change in different lights
- Accuracy still limited.
Using an Electric pH Meter to Measure pH
Using an electric pH meter to measure the pH of a solution
The most common pH testing technique among growers is an electric pH meter. It is more expensive than the other two techniques but gives precise results and are not dependend upon the interpretation of a color. There are different sizes and shapes for these meters but in the end they all work the same. They consist of a display an a probe, the probe is emerged into the solution and the display shows the pH.
But with great precision comes great responsibility. The precision and accuray of those devices can deteriorate over time therefore those devices must be calibrated in order to show the correct pH. Some devices must be calibrated weekly others monthly.
If you use an electric pH meter make sure, to properly clean the probe before and after each measurement and also store the probe property to prevent damage.
Advantages:
- Still fairly easy to do
- High accuracy and precision
- Highly Reusable
Disadvantages:
- Needs to be calibrated regularly
- More costly
Calibrating an Electric pH Meter
Calibrating an electric pH meter is straight forward. All you need is:
- Your electric pH meter of course
- pH Buffer Powder:
- Distilled Water
- Small measuring cups
- If necessary a small screwdriver to adjust the pH meter
Now the steps to calibrate are as follows:
- Use the pH buffer poweder with the prescribed amount of distilled water and try also to have the right temperature. All of which is indicated on the buffer powder.
pH Buffer power to calibrate an electric pH meter
Distilled water always has a pH of 7.0 so the powder will change the pH of the solution to exactly the value which is indicated on the packaging.
- Ensure that the pH probe is clean.
- Check with the manual, and immerse the pH meter into the solution and gently stir it around.
- Now depending on your pH meter you either need to use a screwdriver to set the pH of the meter manually to whatever solution you immersed it in. If the manual says something different, of course follow the manual.
- After you calibrated the probe and before emerging into another solution (maybe for a 2-point calibration) always clean the probe with distilled water.
Once you’ve created the calibration solution you can also save it for later if you need to recalibrate the probe again. Of course it’s better if you use new powder of calibration solution everyday. But properly stored you can reuse calibration solution.
Continuous Monitoring Systems
Apart from the previously shown methods there is also the possibility to continously monitor the pH level of your hydroponic system. The setup works similar to the elctric pH meter, there needs to be a calibration before using. But once calibrated the system provides continous insights into the pH on a near real-time level. This method improves greatly the amount and precision of measurements you are getting. Also because the measurements can be seen and analyzed in real time the grower can also be alerted at any time and act accordingly.
The Hydroplanner is a system which allows you to automatically monitor all of your vital hydroponics metrics and analyze them conveniently via a dashboard.
The hydroplanner dashboard providing insights about pH and TDS
Advantages:
- Once set up gather measurements regularly without further intervention
- Analyze measurements in real time and act upon
- See continous changes also of pH adjustments (see next chapter)
Disadvantages:
- More expensive than previous methods
Adjusting pH Levels
Now we’ve learned what pH is, and why it’s important, moreover we covered how pH can be measured. So you might wonder: “what can I do if the pH is off, and my plants are not getting the nutrients they need to”.
Well of course you can adjust the pH. You can add pH DOWN (pHospHoric acid) and pH UP (potassium hydroxidde) solution to your reservoir and hence decrease or increase the overall pH. Both solutions are just strong acids/alkalines respectively.
pH up and pH down, both used to adjust the pH of a hydroponic solution
Be careful with the amount of those two solutions as they have a very strong effect with only a tiny amount. So make your experiences and start with just drops (depending on your reservoir size of course).
Another major advantage of a continous monitoring system is that you also see the effects of adjusting the pH level. With the other more manual methods, it’s easy to miss the effect you tried to achieve when adjusting the pH. So whenever you adjust the pH it’s recommended to properly stir and mix the water and check again after a couple of minutes and check in short intervals when the final pH level is set and there are no changes anymore. Continuous monitoring system shine here, because all you need to do is, drop some of the solution into your reservoir and then check the dashboards for regular updates. Once the readings are stable you now, whether your pH target was achieved or not.
Why does the pH Level Changes by Itself?
pH not only affect the hydroponics system the pH is also effect by several other factors in hydroponic systems.
One of these effects is the nutrient uptake in plants. When plants absorb nitrogen from the solution through their roots, they deplete a negative charge of the solution. Similarily if plants absorb potassium they deplete the solution a positive charge. Because the solution must remain neutral the plant gives the solution a similar charged ion to compensate. So when the plant absorbs a positive charged it gives a H3O(+) ion back. Therefore the plant decreased the pH of the solution by absorbing a potassium ion. When absorbing nitrate - the plant exchanges for an OH(-) which increases the solutions pH value.
Another factor is the environment containing growth media like stonewool, gravel or glay pebbles. These media act as a buffer and cause the pH to readjust to the media’s own pH level. In non-hydroponic systems soil does the same and keeps the pH stable. To account for the effect in a media-based system get an pH reading from the reservoir as well as the leachate that drains from your meddia bet which holds the plants. You can further read about this topic, check out Science in Hydroponics
A third factor is algae and bacteria. If you experience the pH levels to rise in the morning and drop later in the day, algae might be the problem. Alage consumes acidic carbon dioxide during the day, and therefore pH levels rise and fall again in the evening. Apart from algae, bacteria decomposing old roots also release acids into the hydroponic solution and therefore changing the pH.
Temperature also affects the pH, therefore tap water increases the pH once it’s coming to room temperature. The reason is that the carbonic acid in the water turns into CO2 when the water temperature rises. And because the CO2 evaporates from the water, the acid is gone and hence the pH level rises.
Lastly, the general hardness of the water used, affects pH by its buffering capacity (KH). The buffering capacity increases with the amount of minerals dissolved in water. The buffering capacity counteracts any endevor to adjust the pH and consume whatever pH Up or Down is poured into the system. Only after the buffering capacity is used completely, the water pH levels change.
Maintaining a Healthy pH Level
to recap on what we’ve learned and apply it for the hydroponic system:
- Understand what pH levels your plants need. Use the Solutionexplorer to find out what the optimal pH level is.
- Check your pH levels daily to understand how your system works and is affected by pH. Especially regarding buffering effects described above.
- If your pH is too low then add pH Up
- If your pH is too high then add pH Down
- When adjusting pH levels, regard the buffering capacity.
Have fun growing!